The CO2 Extraction Method
CO2 extraction is one of the safest and cleanest methods and can be used to produce a variety of end products. It can be used to extract CBD, THC, and other cannabinoids from hemp or cannabis while preserving the terpenes contained in the plant material.

CO2 extraction is one of the safest and cleanest methods and can be used to produce a variety of end products. CO2 acts like a solvent at certain temperatures and appropriate pressure. It can be used to extract CBD, THC, and other cannabinoids from hemp or cannabis while preserving the terpenes contained in the plant material.
Although this method is highly expensive and complex, it meets high quality standards. In the food industry, large-scale commercial CO2 extraction plants are often found operating, for example, to decaffeinate coffee and produce essential oils from a variety of plants. CO2 itself is used as an additive in a variety of popular foods and beverages. Carbonated soft drinks, for example, are produced with the help of CO2.
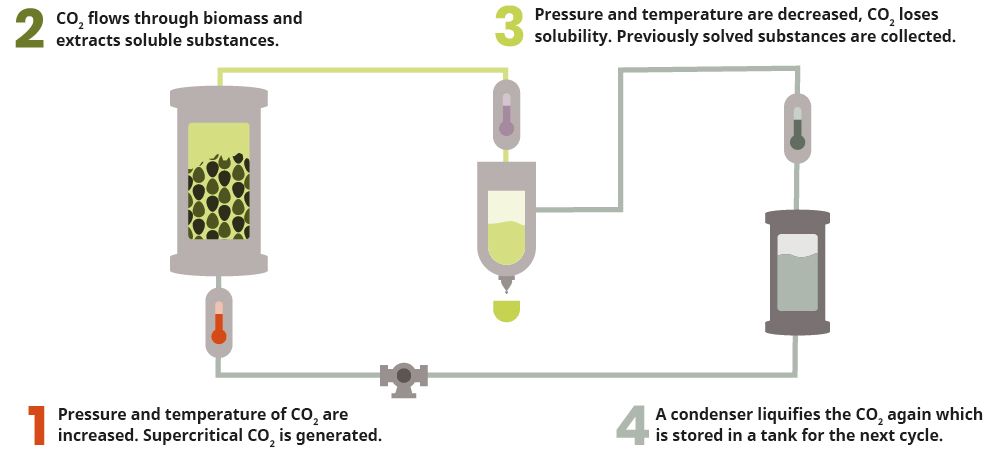
A general distinction is made between supercritical, subcritical and medium-critical CO2 extraction. The supercritical variant proves to be by far the most commonly used CO2 method for the extraction of cannabinoid derivatives, as it is safe and yields a pure end product. In CO2 extraction, one creates phase or aggregate state changes in carbon dioxide using controlled temperatures and pressure. The extraction process begins with the conversion of CO2 gas into a liquid. This is achieved by lowering the temperature while increasing the pressure.
The next step is to raise the temperature by adding pressure and heat beyond the point where the liquid becomes "supercritical." In its "supercritical" state, the CO2 now functions as both a gas and a liquid, making it ideal for chemical extraction. Since it does not damage or denature the substance, it can now be introduced into the plant material for extraction purposes. The CO2 penetrates it, dissolving the membranes of the trichomes and extracting terpenes and cannabinoids as well as CBD and THC. After extraction, the resulting solution is passed through a separator and the desired compounds are separated and collected. The extracted oil is pure, as no trace of the solvent remains in it. It is isolated and preserved to ensure its purity.

CO2 extraction has many advantages for both consumers and extractors and is adaptable to the changing needs of the market. CO2 is non-flammable, non-toxic, chemically inert and leaves virtually no chemical residue. CO2 extraction yields oils with the highest degree of purity and no residual solvents. Cannabinoids produced by this method retain their potency without being contaminated by chlorophyll and other toxic substances. The phase changes in CO2 also make it possible to extract individual parts of the plant material (e.g. cannabinoid derivatives) in different amounts during the extraction process.
Another advantage of CO2 is that its critical temperature is close to room temperature, making it an ideal solvent for temperature-sensitive materials. CO2 is valued for its ability to retain terpenes in the extracted substance. It is therefore ideal for the production of full-spectrum distillates with their associated terpenes.

However, CO2 extraction also has disadvantages. Spent gases are vented to the atmosphere with CO2 extractors, requiring a new solvent for each batch. CO2 extraction systems also operate under high pressure, which is a potential hazard. Consequently, CO2 extraction requires sophisticated equipment and much more practice than ethanol extraction. It takes a lot of specific knowledge and professional training to operate CO2 equipment properly and safely. This is mainly due to the complex phase changes that are necessary during CO2 solvent extraction. These require more in-depth training to ensure a successful outcome. By optimizing the ratios of pressure, temperature and solvent, different cannabinoid derivatives can be extracted by trained extractors.


